|
|
|
이소시아네이트의
자체 반응 (Self-addition reactions)
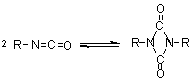
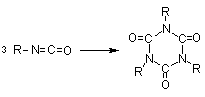
dimerization
(uretidinedione)
trimerization (isocyanurate)
이소시아네이트는 보관중 또는 촉매 존재하에서
2개 이상이 서로 결합되기도 한다.
|
Isocyanate와 hydrogen compound과의
NCO의
반응성
|
Active
hydrogen compound |
Typical
Structure |
Relative
reaction rate* |
|
Primary aliphatic amine |
R-NH2 |
100,000 |
| Secondary
|
RR'NH |
20,000-50,000 |
| Primary aromatic
amine |
Ar-NH2 |
200
- 300 |
| Primary hydroxyl
|
RCH2-OH |
100 |
| Water |
HOH |
100 |
| Carboxilic acid
|
RCOOH |
40 |
| Secondary
hydroxyl |
RR'CHOH |
30 |
| Ureas |
R-NH-CO-NH-R |
15 |
| Tertiary
hydroxyl |
RR'R"C-OH |
0.5 |
| Urethane |
R-NH-CO-O-R |
0.3 |
| Amide |
RCO-NH2 |
0.1 | * uncatalyzed at 25oC
|
TDI 합성법
MDI 합성법

|
|
상세
|